Nuclear Factor Kappa B (NF-κB) is a critical transcription factor involved in regulating the expression of numerous genes that play key roles in immune and inflammatory responses, cell proliferation, and survival. It is present in the cytoplasm of cells and becomes activated in response to various stimuli, such as stress, cytokines, free radicals, and pathogens. Upon activation, NF-κB translocates to the nucleus, where it influences the transcription of genes involved in inflammation, immunity, cell growth, and apoptosis. NF-κB is implicated in a wide range of diseases, including cancer, inflammatory conditions, and neurodegenerative disorders like Parkinson’s disease, where it can act as a double-edged sword by promoting both cell survival and apoptosis depending on the context.
Basic structure and components of Nuclear Factor Kappa B
Explanation of the NF-κB Family
The NF-κB family consists of five transcription factors: RelA (p65), RelB, c-Rel, p50, and p52. These proteins play crucial roles in regulating immune and inflammatory responses, cell survival, and proliferation. RelA (p65) is a critical transactivating component of the NF-κB complex, often forming heterodimers with p50 to regulate gene expression. RelB and c-Rel are involved in distinct signaling pathways, with c-Rel playing a key role in neuron survival and immune tolerance. The p50 and p52 proteins are derived from precursor proteins p105 and p100, respectively, and can form homodimers or heterodimers with other NF-κB members to modulate gene expression. Each member of the NF-κB family has unique and overlapping functions, contributing to the complexity and versatility of NF-κB signaling.
Structural Features of NF-κB Proteins
NF-κB proteins share a conserved Rel homology domain (RHD) responsible for DNA binding, dimerization, and interaction with IκB inhibitors. The C-terminal regions of these proteins vary, contributing to their functional diversity. For instance, the C-terminal domain of RelA (p65) contains two transactivation domains (TA1 and TA2), with TA1 being unstructured but capable of adopting an alpha-helical conformation under certain conditions. The p50 and p52 proteins lack transactivation domains and primarily function as transcriptional repressors unless they form heterodimers with other NF-κB members. RelB contains a bipartite nuclear localization signal (NLS) that mediates its nuclear import, while c-Rel and p52 have monopartite NLSs. These structural features enable NF-κB proteins to interact with various co-factors and regulatory proteins, allowing precise control over gene expression in response to cellular signals.
Functions of Nuclear Factor Kappa B
Nuclear Factor Kappa B (NF-κB) is a pivotal transcription factor that orchestrates a variety of cellular processes essential for maintaining homeostasis and responding to external stimuli. Its functions extend beyond immune regulation, impacting inflammation, cell survival, and proliferation. Below are the key functions of NF-κB:
1. Regulation of Immune Response
Nuclear factor-kappa B (NF-κB) is a critical transcription factor that plays a central role in regulating immune responses. It is rapidly activated in response to various pathogenic signals, including bacterial and viral infections, and is essential for the transcription of genes encoding immunologically relevant proteins. NF-κB activation involves the phosphorylation and degradation of inhibitory proteins (IκBs), allowing NF-κB to translocate to the nucleus and initiate gene transcription. This process is crucial for the activation and differentiation of immune cells, including T and B lymphocytes, and the production of cytokines and chemokines that mediate immune responses.
2. Role in Cell Survival and Proliferation
NF-κB is also pivotal in regulating cell survival and proliferation. It is involved in the transcription of genes that inhibit apoptosis and promote cell cycle progression. In conditions such as endometriosis, NF-κB activity is upregulated, leading to increased cell proliferation and reduced apoptosis, which contributes to the persistence and growth of endometriotic lesions. This transcription factor’s ability to modulate cell survival pathways makes it a key player in various physiological and pathological processes, including cancer and chronic inflammatory diseases.
3. NF-κB in Stress Responses
NF-κB is activated in response to various stress signals, including oxidative stress and endoplasmic reticulum (ER) stress. In naturally long-lived mice, controlled NF-κB activation is associated with preserved immune function and reduced oxidative stress, suggesting a role in promoting longevity. Conversely, dysregulated NF-κB activation in response to chronic stress can lead to increased inflammation and cellular damage. This highlights the importance of NF-κB in maintaining cellular homeostasis and its potential as a target for interventions aimed at mitigating stress-related damage.
4. Involvement in Inflammation
NF-κB is a key regulator of inflammatory responses, controlling the expression of numerous pro-inflammatory genes, including those encoding cytokines, chemokines, and adhesion molecules. Its activation is a critical step in the inflammatory cascade, contributing to the pathogenesis of various inflammatory diseases such as asthma, COPD, and endometriosis. NF-κB’s role in inflammation is further underscored by its involvement in the regulation of inflammasomes and the survival and activation of inflammatory cells. Targeting NF-κB signaling pathways holds therapeutic potential for treating inflammatory conditions.
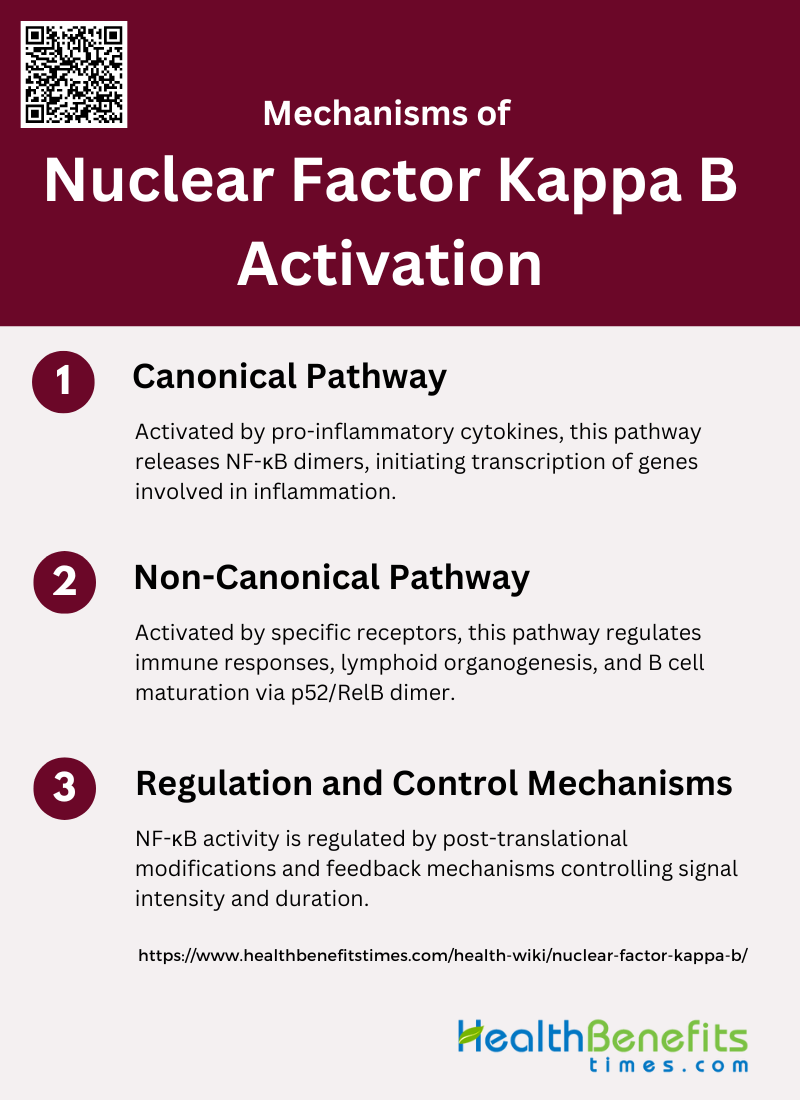
Mechanisms of Nuclear Factor Kappa B Activation
The activation of Nuclear Factor Kappa B (NF-κB) is a complex process that is crucial for its role in regulating immune responses and inflammation. Various stimuli, including cytokines, stress, and pathogens, can trigger its activation. Below are the primary mechanisms through which NF-κB is activated:
1. Canonical Pathway
The canonical pathway of NF-κB activation is primarily triggered by pro-inflammatory cytokines such as TNF-α and IL-1. This pathway involves the activation of the IκB kinase (IKK) complex, which consists of IKKα, IKKβ, and the regulatory subunit IKKγ. Upon activation, IKKβ phosphorylates IκB proteins, leading to their ubiquitination and subsequent proteasomal degradation. This degradation releases NF-κB dimers, typically composed of p65 and p50, which then translocate to the nucleus to initiate transcription of target genes involved in inflammation, immune response, and cell survival.
2. Non-Canonical Pathway
The non-canonical NF-κB pathway is activated by a distinct set of receptors, primarily those in the TNF receptor superfamily, such as BAFF-R, CD40, and LTβR. This pathway is characterized by the stabilization and accumulation of NF-κB-inducing kinase (NIK), which subsequently activates IKKα. Activated IKKα then phosphorylates p100, leading to its processing into p52. The p52/RelB dimer then translocates to the nucleus to regulate genes involved in lymphoid organogenesis, B cell maturation, and immune responses. This pathway is crucial for immune function and its dysregulation is linked to autoimmune diseases and cancers.
3. Regulation and Control Mechanisms
The activity of the NF-κB pathway is tightly regulated through various post-translational modifications, including phosphorylation, ubiquitination, acetylation, sumoylation, and nitrosylation. These modifications can either activate or inhibit NF-κB signaling depending on the context and type of stimulus. For instance, phosphorylation of IκB by IKK leads to its degradation and subsequent activation of NF-κB, while acetylation of NF-κB subunits can enhance their transcriptional activity. Additionally, feedback mechanisms such as the synthesis of IκB proteins, which sequester NF-κB in the cytoplasm, play a crucial role in controlling the duration and intensity of NF-κB signaling.
Nuclear Factor Kappa B in Health and Disease
Nuclear Factor Kappa B (NF-κB) is a critical transcription factor that plays a key role in regulating immune responses, inflammation, and cell survival. Its dysregulation is linked to various diseases, including cancer, chronic inflammatory conditions, and autoimmune disorders. Below is a detailed exploration of NF-κB’s roles in health and disease:
1. Role in Immune System Function
Nuclear factor-kappaB (NF-kappaB) is a pivotal transcription factor in the immune system, regulating the expression of genes involved in both innate and adaptive immunity. It controls the production of cytokines, chemokines, and other molecules essential for immune responses. NF-kappaB activation is triggered by various stimuli, including cytokines, bacterial and viral products, and oxidative stress, which leads to the transcription of genes that mediate immune and inflammatory responses. Dysregulation of NF-kappaB can result in impaired immune function, highlighting its critical role in maintaining immune homeostasis.
2. Impact on Cancer Development
NF-kappaB plays a significant role in cancer development by regulating genes involved in cell proliferation, survival, and apoptosis. Its activation can lead to the expression of anti-apoptotic genes, promoting cell survival and proliferation, which are hallmarks of cancer. Both the canonical and non-canonical pathways of NF-kappaB activation are implicated in various cancers, making it a potential target for cancer therapy. Aberrant NF-kappaB signaling is associated with several malignancies, including lung cancer, breast cancer, and lymphoma, underscoring its importance in oncogenesis.
3. Influence on Chronic Inflammatory Diseases
NF-kappaB is a key regulator of inflammation and is involved in the pathogenesis of chronic inflammatory diseases such as rheumatoid arthritis, inflammatory bowel disease, and asthma. It controls the expression of pro-inflammatory cytokines, chemokines, and adhesion molecules, which are crucial for the inflammatory response. Persistent activation of NF-kappaB leads to chronic inflammation, contributing to tissue damage and disease progression. Targeting NF-kappaB signaling pathways offers potential therapeutic strategies for managing chronic inflammatory conditions.
4. Connection to Autoimmune Diseases
The dysregulation of NF-kappaB is linked to the development of autoimmune diseases, where the immune system mistakenly attacks the body’s own tissues. NF-kappaB regulates genes involved in immune responses and inflammation, and its inappropriate activation can lead to autoimmune conditions such as rheumatoid arthritis, multiple sclerosis, and lupus. By modulating the expression of cytokines and other immune-related genes, NF-kappaB contributes to the pathophysiology of these diseases. Therapeutic strategies targeting NF-kappaB may help in managing autoimmune disorders.
5. Role in Neurodegenerative Disorders
NF-kappaB is implicated in the pathogenesis of neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis. It regulates the expression of genes involved in inflammation, cell survival, and apoptosis, which are critical in the context of neurodegeneration. Chronic activation of NF-kappaB in the central nervous system can lead to neuroinflammation, contributing to neuronal damage and disease progression. Targeting NF-kappaB signaling pathways may offer therapeutic benefits in treating neurodegenerative disorders.
6. Impact on Viral Infections
NF-kappaB plays a crucial role in the host response to viral infections, including HIV and hepatitis. It is activated by viral proteins and contributes to the expression of antiviral genes and inflammatory cytokines. However, some viruses exploit NF-kappaB signaling to enhance their replication and persistence. For instance, HIV-1 utilizes NF-kappaB to promote the transcription of its genome, facilitating viral replication. Understanding the dual role of NF-kappaB in viral infections can aid in developing strategies to modulate its activity for therapeutic purposes.
Therapeutic Targeting of Nuclear Factor Kappa B
Overview of Therapeutic Strategies
Therapeutic strategies targeting Nuclear Factor Kappa B (NF-κB) involve inhibiting its activation and function to mitigate its role in various diseases, including cancer, inflammatory conditions, and viral infections. NF-κB is a transcription factor that regulates genes involved in cell proliferation, survival, and immune responses. Strategies include the use of kinase inhibitors, such as Go6976, which have shown efficacy in reducing tumor growth and inducing tumor regression in estrogen receptor-negative breast cancer models. Additionally, targeting the NF-κB signaling pathway has shown promise in treating lung cancer by enhancing the effects of chemotherapy and radiotherapy. In thyroid cancers, inhibiting NF-κB, alone or in combination with other pathway inhibitors, has demonstrated significant therapeutic benefits.
Drugs Targeting NF-κB
Several drugs have been developed to target NF-κB at different stages of its activation pathway. These include antioxidants like N-Acetyl-L-cysteine (NAC) and alpha-Lipoic acid, which counteract oxidative stress conditions that activate NF-κB. Inhibitors of IκB phosphorylation and degradation, such as salicylates (e.g., aspirin), prevent the release of NF-κB from its inhibitor, thereby blocking its nuclear translocation. Additionally, specific inhibitors like aurine tricarboxylic acid (ATA) have been identified to inhibit NF-κB DNA binding. These drugs have shown potential in various settings, including cancer, HIV, and inflammatory lung diseases.
Potential Benefits and Risks
Targeting NF-κB offers several potential benefits, including reduced tumor growth, enhanced sensitivity to chemotherapy and radiotherapy, and decreased inflammation. For instance, inhibiting NF-κB in lung cancer can potentiate the effects of existing treatments and reduce tumor resistance. In breast cancer, blocking NF-κB activation not only inhibits cell proliferation but also antagonizes its anti-apoptotic role, leading to tumor regression. However, there are risks associated with inhibiting NF-κB, such as potential immunosuppression and unintended effects on normal cell functions, given NF-κB’s role in immune and inflammatory responses. Therefore, careful consideration and targeted approaches are necessary to minimize adverse effects.
Future Research Directions
Future research should focus on developing more specific and potent NF-κB inhibitors with minimal side effects. Investigating the role of non-coding RNAs (ncRNAs) in modulating NF-κB signaling could provide new therapeutic targets, as ncRNAs can either inhibit or promote NF-κB activity, affecting cancer progression and treatment response. Additionally, exploring combination therapies that target multiple pathways involved in tumorigenesis and resistance mechanisms could enhance therapeutic efficacy. Further studies are also needed to understand the long-term effects of NF-κB inhibition and to identify biomarkers for predicting treatment response and monitoring disease progression.