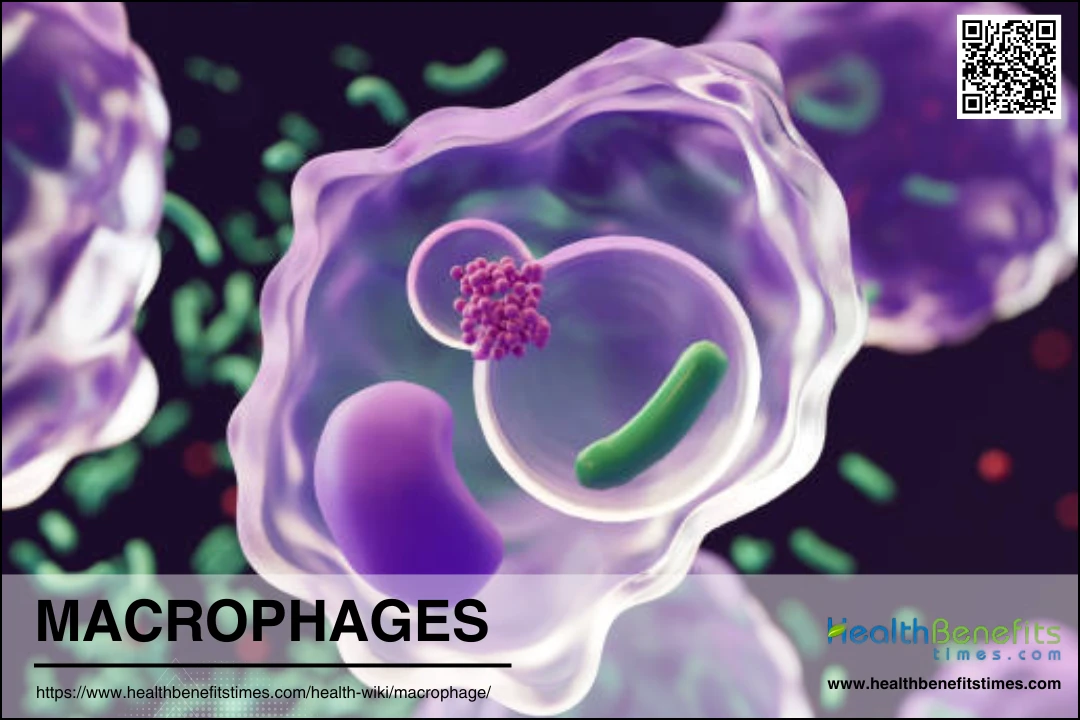
Macrophages are a type of white blood cell that play a crucial role in the immune system by engulfing and digesting cellular debris, foreign substances, microbes, and cancer cells. These versatile cells are found in virtually all tissues and are derived from monocytes, a type of blood cell that migrates into tissues where it differentiates into macrophages. Macrophages are highly adaptable and can change their function based on signals from their environment, which allows them to participate in a wide range of immune responses. They can be broadly categorized into two phenotypes: M1 macrophages, which are pro-inflammatory and help to initiate and sustain immune responses, and M2 macrophages, which are anti-inflammatory and aid in tissue repair and the resolution of inflammation. This adaptability and diversity make macrophages key players in maintaining tissue homeostasis and responding to infections and injuries.
Macrophage Role in the immune system
Macrophages are versatile cells of the innate immune system that play crucial roles in both host defense and tissue homeostasis. They are adept at phagocytosing bacteria, dead cells, and debris, thereby maintaining tissue integrity and responding to infections. Macrophages also act as a bridge between innate and adaptive immunity, influencing immune tolerance, inflammation, and the resolution of tissue damage. Their ability to secrete cytokines and other mediators allows them to modulate immune responses and promote either pro-inflammatory or anti-inflammatory states, depending on the context. Additionally, macrophages are involved in various physiological processes, including angiogenesis, fibrosis, and metabolic regulation, highlighting their importance in both health and disease. Dysfunction in macrophage activity can lead to autoimmune diseases and chronic inflammation, underscoring their critical role in immune system regulation.
Types of Macrophages
They can be categorized based on their origin, activation state, and specialized functions. Here, we explore the different types of macrophages and their unique roles in the body.
1. Resident Macrophages
Resident macrophages are specialized immune cells that reside in specific tissues and perform essential homeostatic and immune functions. Examples include Kupffer cells in the liver, which scavenge blood particles and dying red blood cells, and alveolar macrophages in the lungs, which uptake surfactant and remove airborne pollutants and microbes. These cells are established prenatally from yolk sac-derived erythro-myeloid progenitors and are maintained through self-renewal, independent of adult hematopoiesis. They play crucial roles in maintaining tissue integrity, responding to injury, and supporting tissue-specific functions.
2. Monocyte-derived Macrophages
Monocyte-derived macrophages originate from circulating monocytes that differentiate into macrophages upon entering tissues, especially during inflammation or injury. This differentiation is triggered by environmental signals such as chemokines and cytokines released during tissue damage or infection. These macrophages are crucial for immune responses, as they can rapidly accumulate at sites of injury, promote inflammation, and aid in tissue repair. They exhibit distinct phenotypic and functional properties compared to tissue-resident macrophages, contributing to immune regulation and tissue homeostasis.
3. Activated Macrophages
Activated macrophages are differentiated into two main types: M1 and M2 macrophages. M1 macrophages, or classically activated macrophages, are induced by pro-inflammatory signals and are involved in pathogen elimination and promoting inflammation. M2 macrophages, or alternatively activated macrophages, are induced by anti-inflammatory signals and are involved in tissue repair and resolution of inflammation. These macrophages play diverse roles in immune responses, with M1 macrophages being more pro-inflammatory and M2 macrophages being more involved in healing and immune regulation.
4. Specialized Macrophages
Specialized macrophages are adapted to specific tissues and perform unique functions essential for the homeostasis of those tissues. Examples include osteoclasts in bones, which are involved in bone resorption, and microglia in the central nervous system, which support neuronal circuit development and respond to neural damage. These macrophages are crucial for the proper functioning of their respective tissues and systems, contributing to both normal physiology and responses to injury or disease.
Functions of Macrophages
Macrophages are versatile immune cells that play a vital role in maintaining health and combating disease. They are involved in processes such as pathogen elimination, tissue repair, and immune regulation. Below, we explore the specific functions of macrophages in different contexts.
1. Phagocytosis
Phagocytosis is a critical function of macrophages, involving the engulfment and digestion of pathogens, dead cells, and debris. This process is facilitated by cell surface receptors that recognize and bind to target cells, leading to their internalization into a phagosome. The phagosome then matures, resulting in the destruction and recycling of its contents. This function is essential for innate immunity and tissue homeostasis, as macrophages help clear cellular debris and maintain a clean internal environment.
2. Antigen Presentation
Macrophages play a pivotal role in antigen presentation, which is crucial for activating other immune cells, particularly T-cells. After phagocytosing pathogens, macrophages process and present antigens on their surface in the context of MHC molecules. This interaction with T-cells is vital for the adaptive immune response, as it helps in the recognition and elimination of pathogens. The ability of macrophages to present antigens links the innate and adaptive immune systems, ensuring a coordinated immune response.
3. Cytokine Production
They secrete a variety of pro-inflammatory cytokines, such as TNF-α, IL-1β, IL-6, IL-8, and IL-12, which enhance vascular permeability and recruit other immune cells to the site of infection or injury. Additionally, macrophages produce anti-inflammatory cytokines like IL-10 and TGF-β, which help suppress inflammation and promote tissue repair. This cytokine production is crucial for orchestrating the type and magnitude of the immune response.
4. Immune Surveillance
Macrophages continuously monitor the body for signs of infection or damage, a process known as immune surveillance. They are strategically located throughout body tissues, where they can rapidly respond to changes in the local environment. By ingesting and processing foreign materials, dead cells, and debris, macrophages help maintain tissue homeostasis and prevent the spread of infections. Their ability to adapt to different microenvironments allows them to perform various protective and regulatory functions.
Role of Macrophages in Health and Disease
Macrophages are versatile immune cells that play critical roles in maintaining health and contributing to disease. They are involved in various physiological processes, from immune response and tissue repair to inflammation and cancer. Below are key areas where macrophages significantly impact health and disease:
1. Immune Response and Homeostasis
Macrophages are pivotal in maintaining immune response and homeostasis. They exist in two main subsets: M1 macrophages, which are pro-inflammatory and activated by stimuli such as lipopolysaccharide (LPS) and Th1 cytokines, and M2 macrophages, which are anti-inflammatory and activated by Th2 cytokines like IL-4 and IL-13. M1 macrophages produce pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α, crucial for pathogen destruction. Conversely, M2 macrophages secrete anti-inflammatory cytokines like IL-10 and TGF-β, aiding in tissue repair and homeostasis. The balance between these subsets is essential for immune regulation and preventing chronic inflammation.
2. Tissue Repair and Regeneration
They clear cellular debris, promote angiogenesis, and support stem/progenitor cells, facilitating tissue regeneration. During the initial phase of injury, M1 macrophages dominate to clear pathogens and debris. As healing progresses, M2 macrophages take over, secreting factors that promote tissue repair and remodeling. This transition from M1 to M2 is crucial for effective healing and preventing chronic inflammation or fibrosis. Understanding the mechanisms of macrophage-mediated tissue repair can lead to improved therapeutic strategies for regenerative medicine.
3. Inflammation and Chronic Diseases
In chronic diseases like rheumatoid arthritis (RA) and allergic asthma, an imbalance in macrophage polarization leads to persistent inflammation and tissue damage. In RA, increased M1 macrophages drive chronic inflammation and joint destruction, while in asthma, M2 macrophages contribute to airway remodeling and hyperresponsiveness. Targeting macrophage polarization and function is a promising therapeutic approach for managing chronic inflammatory diseases. Modulating macrophage activity can help restore balance and resolve chronic inflammation.
4. Cancer
Macrophages, particularly tumor-associated macrophages (TAMs), play a dual role in cancer. While they can exhibit anti-tumor activity by promoting immune responses, TAMs often adopt an M2-like phenotype that supports tumor growth, angiogenesis, and metastasis. The tumor microenvironment influences macrophage polarization, leading to a predominance of pro-tumor M2 macrophages. Targeting TAMs to reprogram them towards an anti-tumor M1 phenotype or inhibiting their recruitment to tumors is a potential strategy for cancer therapy. Understanding the complex interactions between macrophages and tumor cells is crucial for developing effective cancer treatments.
5. Metabolic Disorders
Macrophages are implicated in the pathogenesis of metabolic disorders such as obesity and diabetes. In obesity, macrophages infiltrate adipose tissue and adopt a pro-inflammatory M1 phenotype, contributing to insulin resistance and metabolic dysfunction. Conversely, M2 macrophages help maintain metabolic homeostasis by promoting anti-inflammatory responses and tissue repair. The metabolic state of macrophages influences their polarization and function, linking immunometabolism to disease progression. Therapeutic strategies targeting macrophage metabolism and polarization could help manage metabolic disorders and improve metabolic health.
6. Autoimmune Diseases
In autoimmune diseases, macrophages contribute to both disease initiation and progression. They are involved in the regulation of inflammatory responses and tissue repair, but their dysregulation can lead to chronic inflammation and tissue damage. For instance, in rheumatoid arthritis, an imbalance between pro-inflammatory M1 and anti-inflammatory M2 macrophages exacerbates joint inflammation and destruction. Understanding the role of macrophages in autoimmune diseases can lead to the development of targeted therapies that modulate their activity to reduce inflammation and promote tissue repair. Identifying specific macrophage subsets involved in different autoimmune diseases is crucial for designing effective treatments.
FAQs
1. What signals activate macrophages?
Macrophages are activated by various signals, including microbial products (e.g., lipopolysaccharides), cytokines (e.g., IFN-γ, IL-4), and damage-associated molecular patterns (DAMPs) from injured tissues.
2. How do macrophages distinguish between healthy and infected or damaged cells?
Macrophages use pattern recognition receptors (PRRs) to identify pathogen-associated molecular patterns (PAMPs) on microbes and DAMPs on damaged cells, allowing them to differentiate between healthy cells and those that need to be removed.
3. Can macrophages become dysfunctional, and what are the consequences?
Yes, macrophages can become dysfunctional, leading to chronic inflammation, autoimmune diseases, or impaired immune responses. Dysfunctional macrophages are often associated with conditions like atherosclerosis, chronic wounds, and cancer.
4. What is the lifespan of a macrophage?
The lifespan of a macrophage varies depending on its environment and function. Tissue-resident macrophages can live for months to years, while those derived from monocytes during an immune response may only last a few days to weeks.
5. How do macrophages contribute to chronic inflammation?
Macrophages can contribute to chronic inflammation by persistently releasing pro-inflammatory cytokines and failing to switch to an anti-inflammatory or tissue-repair phenotype, leading to ongoing tissue damage.
6. Do macrophages interact with other immune cells?
Yes, macrophages interact with various immune cells, including T-cells, B-cells, and dendritic cells, through cytokine signaling and antigen presentation, influencing both innate and adaptive immune responses.
7. Can macrophages change their function over time?
Yes, macrophages are highly plastic and can change their function in response to different environmental cues, such as transitioning from an inflammatory (M1) to a healing (M2) phenotype during the course of an immune response.
8. What is the difference between macrophages and other phagocytes, like neutrophils?
While both macrophages and neutrophils are phagocytes, macrophages are long-lived, can present antigens, and are involved in both initiating and resolving inflammation, whereas neutrophils are short-lived and primarily involved in the early stages of the immune response.
9. Are macrophages involved in tissue regeneration?
Yes, macrophages play a crucial role in tissue regeneration by clearing debris, promoting angiogenesis, and secreting growth factors that support tissue repair and remodeling.
10. Can macrophages be targeted for therapeutic purposes?
Yes, macrophages are being targeted in therapies for cancer, chronic inflammation, and autoimmune diseases, either by modulating their activity or altering their polarization to promote healing and reduce harmful inflammation.