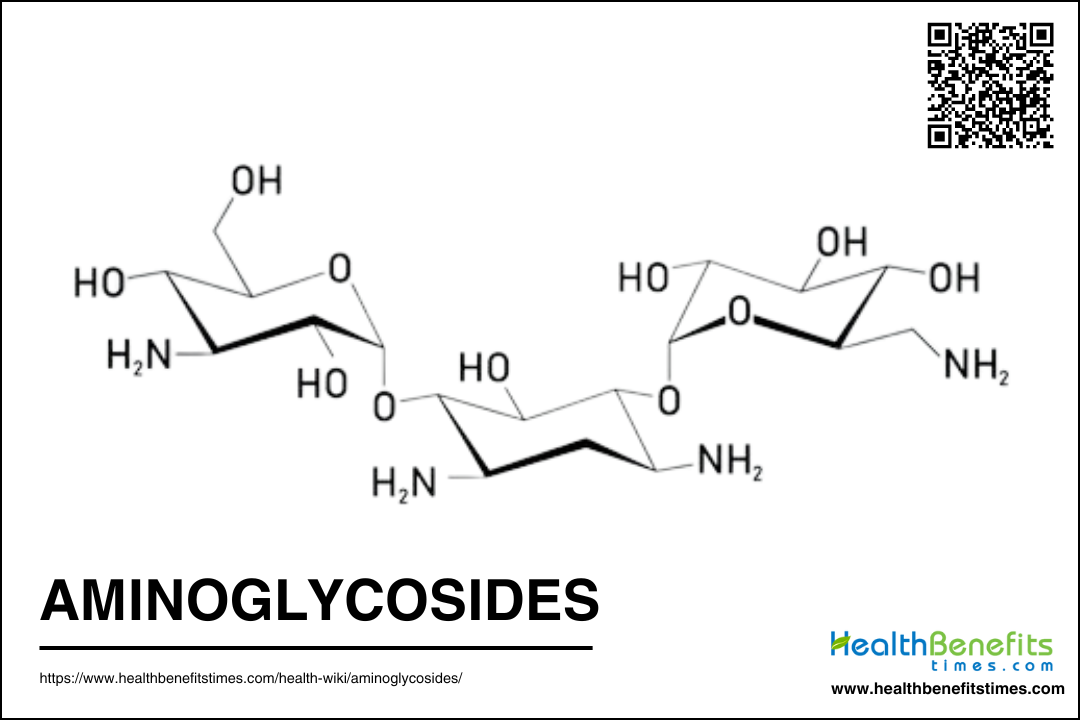
Aminoglycosides are a class of antibiotics known for their effectiveness against aerobic Gram-negative bacteria and certain Gram-positive bacteria. These antibiotics work by binding to the 30S subunit of bacterial ribosomes, thereby inhibiting protein synthesis and leading to bacterial cell death. Common aminoglycosides include gentamicin, tobramycin, amikacin, and streptomycin. They are often used to treat severe infections such as sepsis, complicated urinary tract infections, and nosocomial respiratory infections. However, their use is limited by significant side effects, including nephrotoxicity and ototoxicity, which necessitate careful monitoring during treatment.
Types of Aminoglycosides
They are a class of antibiotics used primarily to treat severe bacterial infections. They work by inhibiting protein synthesis in bacteria, making them effective against a wide range of pathogens. Below are some common types of aminoglycosides:
1. Streptomycin
Streptomycin is one of the earliest discovered aminoglycosides and has been extensively used to treat various bacterial infections. However, its use has become limited due to the availability of newer antibiotics with broader spectrums of activity and fewer side effects. Streptomycin is particularly effective against Mycobacterium tuberculosis and is often used in combination with other antibiotics to treat tuberculosis. Despite its efficacy, streptomycin’s nephrotoxicity and ototoxicity have restricted its use to specific clinical situations where other antibiotics are not effective.
2. Gentamicin
Gentamicin is a widely used aminoglycoside antibiotic effective against a broad spectrum of gram-negative bacteria, including Pseudomonas aeruginosa. It is often used in hospital settings to treat severe infections such as sepsis and pneumonia. Gentamicin is known for its rapid bactericidal action but is also associated with nephrotoxicity and ototoxicity, which necessitates careful dosing and monitoring. Studies have shown that once-daily dosing of gentamicin can reduce nephrotoxicity compared to continuous infusion, making it a safer option for patients.
3. Tobramycin
Tobramycin is another aminoglycoside that is particularly effective against Pseudomonas aeruginosa, making it a common choice for treating infections in cystic fibrosis patients. Like gentamicin, tobramycin is associated with nephrotoxicity and ototoxicity, but once-daily dosing has been shown to mitigate these risks. Tobramycin can be administered via inhalation for respiratory infections, providing high local concentrations in the lungs while minimizing systemic toxicity.
4. Amikacin
Amikacin is often used as a last-resort aminoglycoside when resistance to gentamicin and tobramycin is suspected. It is effective against a wide range of gram-negative bacteria, including multi-drug resistant strains. Amikacin achieves higher serum levels than gentamicin and tobramycin, which can be advantageous in severe infections. Despite its efficacy, amikacin’s nephrotoxic and ototoxic potential requires careful monitoring of serum levels and renal function during treatment.
5. Neomycin
Neomycin is primarily used for topical applications and oral administration for bowel decontamination before surgery due to its high toxicity when administered systemically. It is effective against a variety of gram-negative bacteria but is limited by its nephrotoxicity and ototoxicity. Neomycin is also used in treating hepatic encephalopathy by reducing the number of ammonia-producing bacteria in the gut.
6. Kanamycin
Kanamycin is used to treat serious gram-negative infections, particularly when Pseudomonas aeruginosa is not a likely causative agent. Its use has declined due to the development of resistance and the availability of less toxic alternatives. Kanamycin is still employed in specific clinical situations, such as multi-drug resistant tuberculosis, where it is used in combination with other antibiotics.
7. Plazomicin
Plazomicin is a newer aminoglycoside designed to overcome resistance mechanisms that affect older aminoglycosides. It is effective against a broad range of multi-drug resistant gram-negative bacteria, including carbapenem-resistant Enterobacteriaceae. Plazomicin has shown promise in clinical trials for treating complicated urinary tract infections and bloodstream infections, offering a valuable option in the fight against antibiotic resistance.
8. Paromomycin
Paromomycin is primarily used to treat parasitic infections such as amoebiasis and leishmaniasis. It is also used as an alternative treatment for hepatic encephalopathy. Paromomycin’s use in bacterial infections is limited due to its toxicity and the availability of more effective antibiotics. However, it remains a valuable option in specific parasitic infections where other treatments are not effective.
9. Netilmicin
Netilmicin is similar to gentamicin and tobramycin but is less commonly used. It is effective against a broad spectrum of gram-negative bacteria and is often reserved for cases where resistance to other aminoglycosides is suspected. Netilmicin has a slightly lower nephrotoxic potential compared to gentamicin and tobramycin, making it a safer option in some cases.
Benefits of Aminoglycosides
They are powerful antibiotics that offer several advantages in treating bacterial infections. They are particularly effective against gram-negative bacteria and are often used in combination with other antibiotics to enhance their efficacy. Below are some key benefits of aminoglycosides:
1. Broad-Spectrum Activity
Aminoglycosides are renowned for their broad-spectrum antibacterial activity, making them highly effective against a wide range of bacterial pathogens. These antibiotics work by binding to the bacterial 16S rRNA, thereby disrupting protein synthesis and leading to bacterial cell death. This broad-spectrum activity is particularly valuable in treating severe infections where the causative agent is not immediately known. The ability of aminoglycosides to target both Gram-positive and Gram-negative bacteria, including resistant strains, underscores their importance in clinical settings, especially in an era of increasing antibiotic resistance.
2. Synergistic Effects
This synergism is particularly notable with beta-lactam antibiotics, which disrupt bacterial cell wall synthesis, allowing aminoglycosides to more effectively penetrate and inhibit protein synthesis. This combination therapy is often employed in the treatment of serious infections, such as those caused by multidrug-resistant bacteria, where monotherapy may not be sufficient. However, it is crucial to use these combinations judiciously to avoid unnecessary side effects and resistance development.
3. Low Resistance Potential
Despite decades of clinical use, aminoglycosides have maintained a relatively low resistance potential compared to other antibiotic classes. This is partly due to their unique mechanism of action and the difficulty bacteria face in mutating the 16S rRNA target site without compromising their own viability. Additionally, the development of semisynthetic aminoglycosides has further mitigated resistance issues by overcoming common resistance mechanisms. This low resistance potential makes aminoglycosides a valuable option in the treatment of infections caused by resistant bacterial strains.
4. Serious Infection Management
Their rapid bactericidal action and broad-spectrum activity make them particularly effective in these high-stakes scenarios. For instance, in cases of sepsis, aminoglycosides can quickly reduce bacterial load, buying time for other treatments to take effect. Their role in treating severe nosocomial infections, especially those caused by multidrug-resistant organisms, further highlights their importance in modern medicine.
5. Cystic Fibrosis Treatment
In patients with cystic fibrosis, aminoglycosides are used to manage chronic respiratory infections caused by Pseudomonas aeruginosa and other resistant pathogens. These antibiotics are often administered via inhalation, which allows for high local concentrations in the lungs while minimizing systemic toxicity. The ability of aminoglycosides to disrupt biofilms and their potent bactericidal activity make them particularly effective in this context. This targeted approach helps to manage persistent infections and improve the quality of life for cystic fibrosis patients.
6. Tuberculosis Treatment
They are particularly valuable in managing multidrug-resistant TB (MDR-TB) and extensively drug-resistant TB (XDR-TB) cases, where other antibiotics may fail. The bactericidal action of aminoglycosides helps to rapidly reduce the bacterial load, which is crucial in preventing the spread of TB and improving patient outcomes. Despite their potential for toxicity, the benefits of aminoglycosides in treating severe TB cases often outweigh the risks, making them indispensable in TB management.
Clinical Uses of Aminoglycosides
They are particularly effective against gram-negative bacteria and are often employed in combination with other antibiotics for enhanced efficacy. Below are some key clinical uses of aminoglycosides:
1. Severe Gram-Negative Infections
Aminoglycosides are highly effective against severe Gram-negative infections, particularly those caused by multidrug-resistant pathogens such as Pseudomonas aeruginosa and Acinetobacter spp. These antibiotics exhibit concentration-dependent bacterial killing, making them valuable in treating serious infections. Extended-interval dosing schemes are often recommended to maximize efficacy and minimize toxicity, such as nephrotoxicity and ototoxicity. Despite their potential side effects, aminoglycosides remain a crucial part of the antimicrobial arsenal, especially in cases where other antibiotics may fail due to resistance.
2. Respiratory Infections
These antibiotics are effective against Pseudomonas aeruginosa, a common pathogen in cystic fibrosis patients. However, their use is often limited by the risk of nephrotoxicity and ototoxicity. Strategies such as on-demand drug delivery systems and smart hydrogels are being explored to mitigate these side effects while maintaining high antibacterial activity. The development of new formulations aims to provide targeted and controlled release of aminoglycosides, enhancing their safety profile.
3. Tuberculosis
Streptomycin, an aminoglycoside, was one of the first antibiotics used to treat tuberculosis. Although its use has declined due to the development of other effective drugs, aminoglycosides are still employed in multidrug-resistant tuberculosis cases. The resurgence of interest in aminoglycosides is partly due to their ability to overcome resistance mechanisms. Newer aminoglycosides like plazomicin have been developed to address resistance issues, marking a rejuvenation of this antibiotic class.
4. Bone and Joint Infections
They are particularly effective when incorporated into polymethylmethacrylate bone cements, which provide a localized and sustained release of the antibiotic, reducing systemic toxicity. This approach is beneficial in orthopedic surgical infections, where high local concentrations of the antibiotic are required to eradicate the infection. Careful dosing and monitoring are essential to minimize the risk of nephrotoxicity and ototoxicity.
5. Endocarditis
Aminoglycosides are frequently used in combination with beta-lactams or glycopeptides for the treatment of bacterial endocarditis caused by streptococci, staphylococci, and enterococci. However, evidence suggests that their addition does not significantly improve outcomes and may increase the risk of side effects. The use of aminoglycosides in endocarditis requires careful consideration of dosing regimens and duration to balance efficacy and toxicity. Larger clinical trials are needed to refine treatment guidelines and optimize the use of aminoglycosides in endocarditis.
6. Perioperative Prophylaxis
Their rapid bactericidal action and synergistic effects with other antibiotics make them suitable for perioperative prophylaxis. However, the potential for nephrotoxicity and ototoxicity necessitates careful patient selection and monitoring. Single daily dosing regimens are often employed to reduce the risk of toxicity while maintaining efficacy
7. Cystic Fibrosis
In cystic fibrosis patients, aminoglycosides are used to manage chronic respiratory infections caused by Pseudomonas aeruginosa. The antibiotics are administered via inhalation to deliver high local concentrations directly to the lungs, minimizing systemic exposure and reducing the risk of nephrotoxicity and ototoxicity. Innovative drug delivery systems, such as smart hydrogels, are being developed to enhance the therapeutic outcomes and safety of aminoglycosides in cystic fibrosis. These advancements aim to provide more effective and safer treatment options for patients.
8. Bacterial Conjunctivitis
Topical formulations of aminoglycosides, such as eye drops, are commonly used to deliver the antibiotic directly to the site of infection, reducing the risk of systemic side effects. The rapid bactericidal action of aminoglycosides makes them a preferred choice for managing bacterial conjunctivitis . However, the potential for local irritation and allergic reactions should be considered.
9. Complex Abdominal Infections
Their rapid bactericidal activity and synergistic effects with beta-lactams enhance the overall efficacy of the treatment regimen. Extended-interval dosing schemes are often employed to optimize therapeutic outcomes and minimize toxicity. Despite their potential side effects, aminoglycosides remain an important option for managing severe abdominal infections.
Routes of administration (IV, IM, topical) of Aminoglycosides
Aminoglycosides can be administered through various routes to effectively treat different types of bacterial infections. The choice of administration method—intravenous (IV), intramuscular (IM), or topical—depends on the severity and location of the infection. Below are the primary routes of administration for aminoglycosides:
1. Intravenous (IV)
Intravenous (IV) administration of aminoglycosides is a common route for treating systemic infections due to its rapid distribution throughout the extracellular fluid compartment. Peak serum levels are typically achieved within 30 minutes of the end of a 30-minute infusion, making it an effective method for delivering high concentrations of the drug quickly. However, the risk of nephrotoxicity and ototoxicity remains a significant concern, necessitating careful monitoring of serum drug levels to avoid toxic accumulation, especially in patients with impaired renal function. IV administration is often preferred in clinical settings due to its convenience and the ability to control dosing precisely.
2. Intramuscular (IM)
Intramuscular (IM) administration of aminoglycosides is another effective route, particularly useful when IV access is not available. The absorption rate after IM injection is generally faster in neonates compared to adults, and the volume of distribution is significantly larger in this population. IM administration results in peak serum levels that are slightly lower than those achieved with IV administration, but it still provides effective drug concentrations for treating infections. This route is commonly used in outpatient settings or in situations where prolonged IV therapy is impractical. However, similar to IV administration, the risk of nephrotoxicity and ototoxicity necessitates careful monitoring.
3. Topical
Topical administration of aminoglycosides is primarily used for localized infections, such as skin infections or wound infections. This route allows for high local concentrations of the drug with minimal systemic absorption, thereby reducing the risk of systemic toxicity. Aminoglycosides are well absorbed from large wounds and granulating areas when applied topically, making them effective for treating infections in these regions. The use of topical aminoglycosides is advantageous in minimizing the risk of nephrotoxicity and ototoxicity, which are more common with systemic administration.
4. Inhalation
Inhalation of aminoglycosides is particularly useful in treating respiratory infections, such as those caused by Pseudomonas aeruginosa in cystic fibrosis patients. This route allows for direct delivery of the drug to the lungs, achieving high local concentrations while minimizing systemic exposure and associated toxicities. The pharmacokinetics of inhaled aminoglycosides show increased volume of distribution and clearance rates, making them effective for managing chronic lung infections. However, the potential for ototoxicity and nephrotoxicity still exists, necessitating careful monitoring and dose adjustments.
5. Oral
Oral administration of aminoglycosides is generally not effective for treating systemic infections due to poor absorption from the gastrointestinal tract. However, in patients with impaired renal function, prolonged oral administration can lead to toxic accumulation of the drug in the body. Oral aminoglycosides are sometimes used for gut decontamination or to treat hepatic encephalopathy by reducing ammonia-producing bacteria in the gut. Despite their limited use in systemic infections, oral aminoglycosides can be effective in specific clinical scenarios where localized gut action is required.
6. Intraperitoneal
Intraperitoneal administration of aminoglycosides is commonly used in patients undergoing peritoneal dialysis who require antibiotic therapy. This route allows for easy administration and achieves therapeutic plasma concentrations, although continuous therapeutic levels may increase the risk of ototoxicity. The absorption of aminoglycosides from the peritoneal cavity is concentration-dependent, with inflammatory changes in peritonitis significantly increasing absorption rates. While this method is convenient, the potential for audiovestibular damage remains a concern, necessitating careful monitoring of drug levels and patient symptoms.
7. Intraventricular
Intraventricular (IVT) administration of aminoglycosides is used to treat central nervous system (CNS) infections, such as meningitis and ventriculitis, particularly when caused by multidrug-resistant organisms. This route allows for high drug concentrations in the cerebrospinal fluid (CSF), which are difficult to achieve with systemic administration alone. IVT aminoglycosides have been shown to be effective and safe, with no serious adverse effects reported in the literature. However, optimal dosing regimens are unclear, and therapeutic drug monitoring is recommended for more complicated cases. This route is particularly useful in treating infections associated with intracranial devices and neurosurgery prophylaxis.
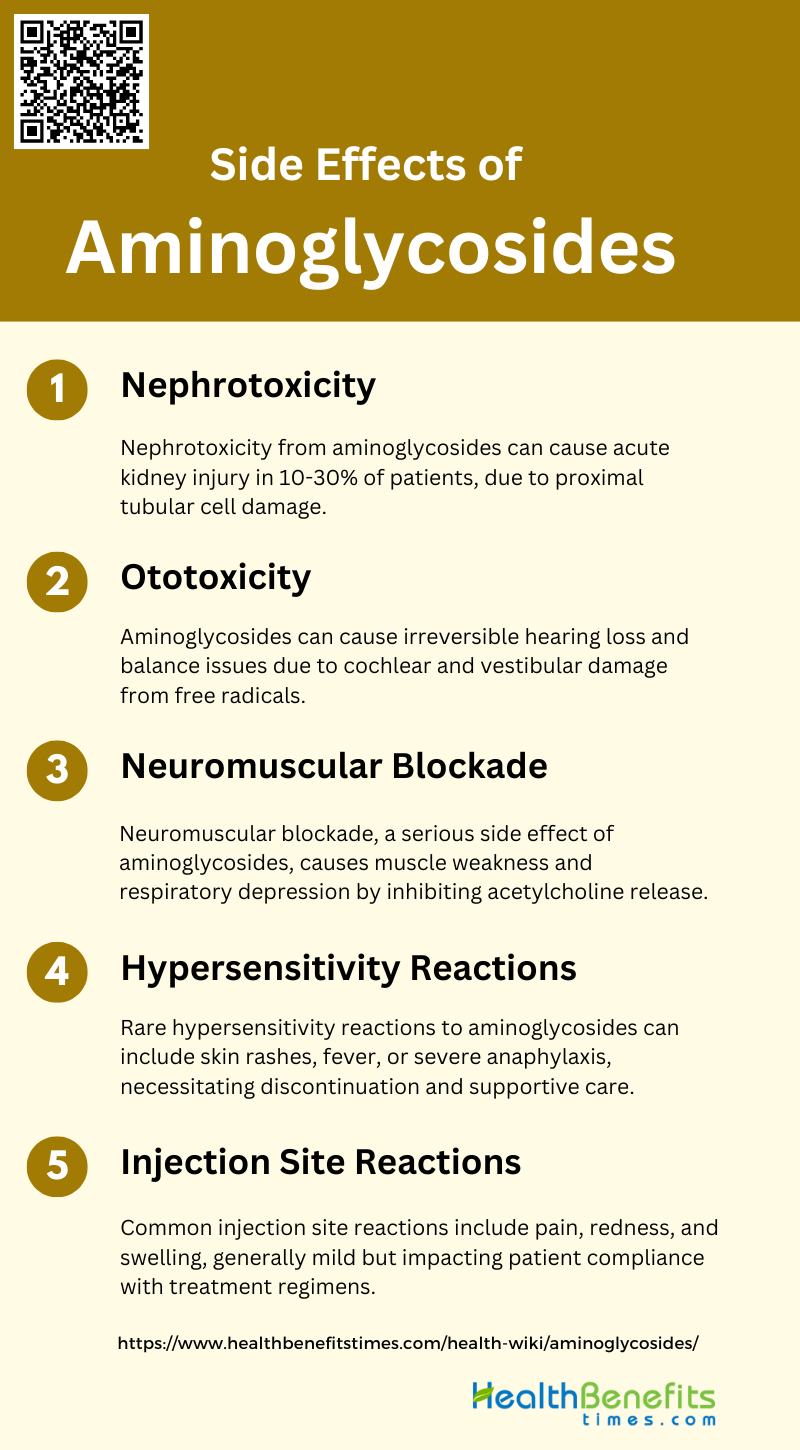
Side Effects of Aminoglycosides
While aminoglycosides are effective antibiotics, they can cause several side effects, particularly with prolonged use or high doses. These side effects can range from mild to severe and may impact various body systems. Below are some common side effects associated with aminoglycosides:
1. Nephrotoxicity
The nephrotoxicity mechanism involves the uptake of the drug by proximal tubular cells, leading to lysosomal phospholipidosis and subsequent cell necrosis. Despite advancements in dosing schedules and monitoring procedures, aminoglycosides remain a significant cause of drug-induced nephrotoxicity. The average frequencies of nephrotoxicity for gentamicin and tobramycin are 14.0% and 12.9%, respectively, while netilmicin and amikacin show slightly lower rates at 8.7% and 9.4%, respectively. Efforts to develop less toxic compounds have met with limited success, emphasizing the need for continued research to improve the safety profile of these essential antibiotics.
2. Ototoxicity
Ototoxicity is another major side effect of aminoglycosides, often resulting in irreversible hearing loss or balance issues. The cochlear damage caused by these drugs can lead to permanent hearing loss, while vestibular damage can result in dizziness and ataxia. The mechanisms underlying ototoxicity include the generation of free radicals within the inner ear and genetic predispositions, such as mutations in the mitochondrial 12S rRNA gene. The average frequency of cochlear toxicity varies among different aminoglycosides, with amikacin showing the highest rate at 13.9%, followed by gentamicin and tobramycin at 8.3% and 6.1%, respectively. Strategies to mitigate ototoxicity include the use of anti-free radical agents like salicylate and reducing cochlear uptake of the drugs.
3. Neuromuscular Blockade
This condition is characterized by muscle weakness and respiratory depression, which can be life-threatening if not promptly managed. The mechanism involves the inhibition of acetylcholine release at the neuromuscular junction, leading to impaired muscle contraction. Although this side effect is less frequently reported compared to nephrotoxicity and ototoxicity, it remains a significant concern, particularly in patients with underlying neuromuscular disorders or those receiving concurrent neuromuscular blocking agents. Preventive measures include careful monitoring of drug levels and avoiding high doses, especially in susceptible individuals.
4. Hypersensitivity Reactions
Hypersensitivity reactions to aminoglycosides are relatively rare but can occur. These reactions may manifest as skin rashes, fever, or more severe forms such as anaphylaxis. The exact mechanism is not well understood but is thought to involve immune-mediated responses to the drug or its metabolites. While hypersensitivity reactions are less common compared to other side effects like nephrotoxicity and ototoxicity, they can still pose significant risks to patients. Management typically involves discontinuation of the offending agent and supportive care, including antihistamines or corticosteroids for milder reactions and epinephrine for anaphylaxis.
5. Injection Site Reactions
Injection site reactions are a common but generally mild side effect of aminoglycoside administration. These reactions can include pain, redness, and swelling at the site of injection. While these symptoms are usually self-limiting and resolve without intervention, they can cause discomfort and may lead to non-compliance with treatment regimens. Strategies to minimize injection site reactions include proper injection techniques, rotating injection sites, and using local anesthetics if necessary. Despite being less severe than nephrotoxicity or ototoxicity, injection site reactions can still impact the overall patient experience and adherence to therapy.