Hemostasis is the physiological process that prevents and stops bleeding, or hemorrhage, by forming a blood clot at the site of vascular injury. It involves a complex interplay of vascular, cellular, and molecular mechanisms. Initially, the coagulation system activates to form a fibrin clot, while platelets adhere to the damaged vessel wall and aggregate to form a hemostatic plug. This process is tightly regulated by various blood-borne inhibitors and feedback loops to ensure that clotting is localized and temporary. Hemostasis is divided into primary hemostasis, which involves platelet-vessel interactions, and secondary hemostasis, which involves the coagulation cascade leading to fibrin clot formation. The balance between procoagulant and anticoagulant forces is crucial to prevent excessive bleeding or thrombosis. Red blood cells contribute to hemostasis by influencing blood viscosity and flow dynamics, and by interacting with platelets and coagulation factors.
Types of hemostasis
Hemostasis is a vital physiological process that prevents and controls bleeding by forming a stable blood clot at the site of vessel injury. It involves a series of complex mechanisms that work together to ensure effective clot formation and wound healing. The types of hemostasis can be categorized as follows:
1. Primary Hemostasis
Primary hemostasis involves the initial response to vascular injury, where platelets play a crucial role. Upon vessel damage, platelets adhere to the exposed collagen and other subendothelial components, facilitated by von Willebrand factor (VWF). This adhesion triggers platelet activation, leading to the release of granules that further recruit and activate additional platelets, forming a temporary “platelet plug” to seal the injury site. This process is tightly regulated by signaling molecules and receptors to ensure that the platelet plug is confined to the site of injury, preventing unnecessary clot formation.
2. Secondary Hemostasis
Secondary hemostasis refers to the coagulation cascade, a series of enzymatic reactions that stabilize the initial platelet plug with a fibrin mesh. This process involves both intrinsic and extrinsic pathways that converge to activate Factor X, leading to the generation of thrombin. Thrombin then converts fibrinogen into fibrin, which forms a stable clot. This phase is regulated by natural anticoagulants like antithrombin and protein C to prevent excessive clotting. The balance between coagulation and anticoagulation is crucial to maintaining hemostatic integrity.
3. Tertiary Hemostasis
Tertiary hemostasis, also known as fibrinolysis, involves the breakdown of the fibrin clot once tissue repair is underway. This process is mediated by plasmin, an enzyme that degrades fibrin into soluble fragments. Plasmin is generated from plasminogen, which is activated by tissue plasminogen activator (tPA). The regulation of fibrinolysis is essential to prevent premature clot dissolution, which could lead to re-bleeding, and to ensure that clots do not persist longer than necessary, which could obstruct blood flow.
4. Chemical/Topical Hemostasis
Chemical or topical hemostasis involves the application of agents directly to the bleeding site to promote clotting. These agents can be natural, synthetic, or a combination of both, and include substances like thrombin, fibrin sealants, and hemostatic dressings. These materials are particularly useful in surgical and emergency settings where rapid hemostasis is required. Advances in this field have led to the development of more effective and targeted hemostatic agents that can be used in various clinical scenarios.
5. Mechanical Hemostasis
Mechanical hemostasis involves the use of physical methods to control bleeding, such as applying direct pressure, using tourniquets, or employing surgical techniques like suturing and ligation. These methods are often the first line of defense in trauma and surgical settings to quickly control hemorrhage. Mechanical hemostasis can be combined with other hemostatic techniques to enhance effectiveness, especially in cases of severe bleeding.
6. Natural Hemostasis
Natural hemostasis refers to the body’s intrinsic ability to stop bleeding through the coordinated actions of platelets, coagulation factors, and the vascular system. This process is finely tuned to respond to vascular injury by forming a clot that is later dissolved once healing is complete. The natural hemostatic process involves a delicate balance between procoagulant and anticoagulant forces to maintain blood fluidity while preventing excessive blood loss.
7. Thermal Hemostasis
Thermal hemostasis involves the use of heat to coagulate blood and tissue, thereby stopping bleeding. This method is commonly employed in surgical procedures using tools like electrocautery and laser coagulation. Thermal hemostasis is effective in controlling bleeding from small vessels and can be used in conjunction with other hemostatic techniques to achieve optimal results. However, it requires careful application to avoid tissue damage.
8. Artificial Hemostasis
Artificial hemostasis encompasses the use of engineered materials and technologies to mimic or enhance the body’s natural hemostatic processes. This includes the development of synthetic platelets, hemostatic nanoparticles, and advanced biomaterials designed to promote clotting and stabilize clots. These innovations are particularly valuable in situations where natural hemostasis is insufficient, such as in patients with bleeding disorders or during complex surgical procedures. The goal is to create effective, safe, and easily deployable hemostatic solutions that can be used in a variety of clinical settings.
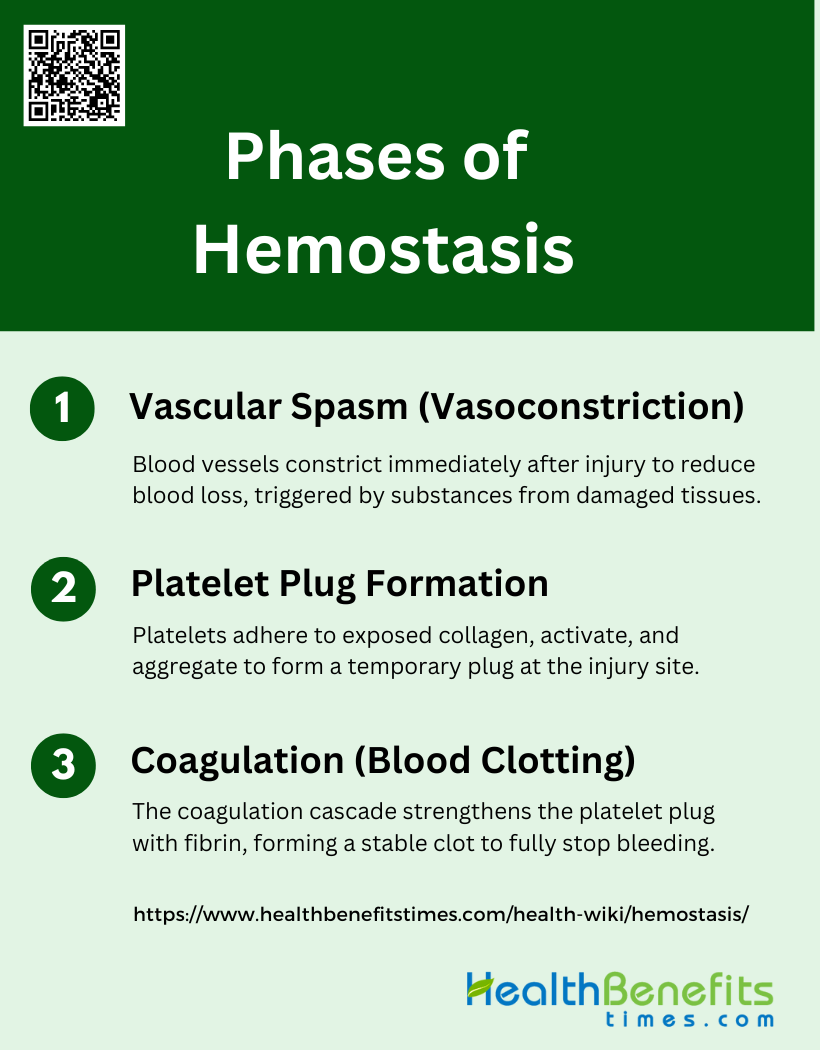
Phases of Hemostasis
Hemostasis is a critical process that prevents and controls bleeding by forming a stable clot at the site of vessel injury. It involves a series of well-coordinated phases that work in tandem to ensure effective clot formation and wound healing. The phases of hemostasis include:
1. Vascular Spasm (Vasoconstriction)
Vascular spasm, or vasoconstriction, is the initial phase of hemostasis, characterized by a brief constriction of blood vessels. This immediate response to vascular injury serves to reduce blood flow to the injury site, thereby minimizing blood loss. The process is triggered by the release of vasoconstrictive substances from the damaged endothelium and surrounding tissues. For instance, when you get a paper cut, the blood vessels in the affected area tighten to prevent excessive blood loss. This phase is crucial as it sets the stage for subsequent hemostatic processes, including platelet plug formation and coagulation.
2. Platelet Plug Formation
Platelet plug formation is the second phase of hemostasis, where platelets adhere to the injured area and to each other, forming a temporary “plug.” This process begins with the adhesion of platelets to exposed collagen fibers at the injury site, followed by their activation and aggregation. Activated platelets release chemicals such as ADP and thromboxane A2, which attract more platelets to the area, amplifying the response. This self-amplifying process is essential for creating a barrier at the cut site, effectively reducing blood loss until a more stable clot can form.
3. Coagulation (Blood Clotting)
Coagulation, or blood clotting, is the third phase of hemostasis, involving the formation of a more permanent clot through a cascade of reactions that result in the production of fibrin. This phase strengthens the initial platelet plug by creating a mesh of cross-linked fibrin strands that stabilize the clot. The coagulation cascade is initiated by tissue factor exposure and involves both intrinsic and extrinsic pathways, culminating in the generation of thrombin. Thrombin converts soluble fibrinogen into insoluble fibrin, which then polymerizes to form a stable clot, effectively stopping the bleeding entirely.
Key Factors Involved in Hemostasis
Hemostasis is a complex process that involves various components working together to prevent and stop bleeding. Each component plays a specific role in ensuring the effective formation and stabilization of a blood clot. The key factors involved in hemostasis include:
1. Platelets
Platelets play a crucial role in hemostasis by rapidly adhering to the site of vascular injury, becoming activated, and aggregating to form a primary hemostatic plug. Upon vascular injury, platelets adhere to the exposed subendothelial extracellular matrix, primarily through interactions with von Willebrand factor (VWF) and collagen. Activated platelets release granules containing various substances that further promote platelet aggregation and recruit additional platelets to the site of injury. This process is essential for the initial cessation of bleeding and provides a surface for the assembly of coagulation factors, which consolidate the platelet plug by forming a fibrin mesh.
2. Clotting Factors
Clotting factors are proteins in the blood plasma that work together in a complex cascade to form a stable blood clot. The coagulation cascade is traditionally divided into the intrinsic and extrinsic pathways, both of which converge on the common pathway leading to the formation of thrombin and fibrin. The extrinsic pathway is initiated by tissue factor (TF) and factor VIIa, while the intrinsic pathway involves factors VIII, IX, and XI. These pathways are tightly regulated to ensure that clotting occurs only at the site of injury. The interaction between clotting factors and platelets is critical, as activated platelets provide a surface for the assembly of these factors, enhancing the efficiency of the coagulation process.
3. Calcium Ions (Factor IV)
Calcium ions, also known as Factor IV, are essential for various steps in the coagulation cascade. They act as cofactors for several enzymes in the cascade, facilitating the binding of clotting factors to phospholipid surfaces, such as those provided by activated platelets. Calcium ions are required for the activation of factors IX and X, as well as for the conversion of prothrombin to thrombin. The presence of calcium is crucial for the structural integrity of the clotting factors and for the proper assembly of the coagulation complexes on the platelet surface, thereby ensuring efficient clot formation.
4. von Willebrand Factor
Von Willebrand factor (VWF) is a large multimeric glycoprotein that plays a key role in both primary and secondary hemostasis. It mediates the adhesion of platelets to the subendothelial matrix at sites of vascular injury and serves as a carrier protein for factor VIII, protecting it from degradation in the circulation. VWF is stored in Weibel-Palade bodies in endothelial cells and is released upon vascular injury. It forms long strings that capture platelets, facilitating their adhesion and activation. The interaction between VWF and factor VIII is critical for the amplification of the coagulation cascade, making VWF essential for effective hemostasis.
5. Vitamin K
Vitamin K is a fat-soluble vitamin that is essential for the post-translational modification of several clotting factors, including factors II (prothrombin), VII, IX, and X. These factors require carboxylation of specific glutamic acid residues to become fully functional, a process that is dependent on vitamin K. Without adequate vitamin K, these clotting factors cannot bind calcium ions effectively, impairing their ability to participate in the coagulation cascade. Vitamin K deficiency can lead to bleeding disorders due to the production of dysfunctional clotting factors, highlighting its critical role in maintaining hemostasis.
6. Endothelial Cells
Endothelial cells line the interior surface of blood vessels and play a pivotal role in hemostasis by regulating the balance between procoagulant and anticoagulant activities. They produce and release von Willebrand factor, which is crucial for platelet adhesion and aggregation. Endothelial cells also express tissue factor, which initiates the extrinsic pathway of coagulation. Additionally, they produce anticoagulant substances such as prostacyclin and nitric oxide, which inhibit platelet aggregation and vasoconstriction. The dynamic interaction between endothelial cells and other components of the hemostatic system ensures that clotting occurs only at the site of injury, preventing excessive bleeding or thrombosis.
Disorders Related to Hemostasis
Hemostasis disorders encompass a range of conditions that disrupt the normal balance of clot formation and dissolution, leading to either excessive bleeding or clotting. These disorders can have significant health implications, requiring careful diagnosis and management to prevent complications. The main categories of disorders related to hemostasis include:
1. Bleeding Disorders
Bleeding disorders are conditions that affect the body’s ability to form blood clots, leading to excessive bleeding. Examples include hemophilia, where deficiencies in clotting factors VIII or IX disrupt thrombin generation, and thrombocytopenia, characterized by low platelet counts that impair primary hemostasis. These disorders disturb the hemostatic balance, making it difficult to control bleeding. Treatment options vary but often include replacement therapies for missing clotting factors, platelet transfusions, and medications to enhance clot formation or reduce bleeding risk.
2. Thrombotic Disorders
Thrombotic disorders involve excessive clotting, which can lead to conditions such as deep vein thrombosis (DVT) and pulmonary embolism (PE). These disorders result from an imbalance in the coagulation system, often due to deficiencies in anticoagulant proteins or mutations like factor V Leiden. The consequences of excessive clotting include blocked blood vessels, which can cause severe cardiovascular events. Management strategies typically involve anticoagulant medications, such as direct oral anticoagulants or vitamin K antagonists, and preventive measures like thromboprophylaxis in high-risk patients.
Role of Hemostasis in Wound Healing
Hemostasis is a vital initial step in the wound healing process, ensuring that bleeding is controlled and the wound site is stabilized. It sets the stage for subsequent healing phases by forming a protective barrier and facilitating tissue repair. The role of hemostasis in wound healing can be understood through the following aspects:
1. Wound Protection
Hemostasis is the first critical phase of wound healing, where the formation of a blood clot serves as a primary defense mechanism against infections. Upon injury, platelets aggregate and activate the coagulation cascade, leading to the formation of a fibrin clot that seals the wound site. This clot acts as a physical barrier, preventing the entry of pathogens and providing a scaffold for incoming leukocytes, which are essential for immune defense. Additionally, fibrinogen within the clot traps bacteria, further enhancing the wound’s protection against microbial invasion. This initial barrier is crucial for maintaining the wound’s sterility and facilitating subsequent healing phases.
2. Wound Repair
Following hemostasis, the body transitions into the inflammation, proliferation, and remodeling phases to repair the damaged tissue. The fibrin clot formed during hemostasis serves as a provisional matrix that supports the migration of various cell types, including fibroblasts and endothelial cells, which are essential for tissue regeneration. Growth factors and cytokines released during clot formation promote cellular activities necessary for wound repair, such as re-epithelialization and angiogenesis. The coordinated action of these cells and molecules ensures the replacement of the damaged tissue with new, functional tissue, ultimately leading to wound closure and restoration of tissue integrity.
3. Scarring
Proper hemostasis is crucial for minimizing scarring during wound healing. The timely formation and resolution of the fibrin clot help to regulate the subsequent phases of healing, particularly inflammation and tissue remodeling. Efficient hemostasis ensures that the inflammatory response is well-controlled, reducing the risk of excessive fibroblast activity and collagen deposition, which are primary contributors to scar formation. Additionally, the clot provides a scaffold that supports organized tissue regeneration, promoting the formation of a competent provisional matrix that facilitates normal tissue architecture and function. By optimizing these processes, proper hemostasis can significantly reduce the extent of scarring and improve the overall quality of wound healing.
Medical Intervention in Hemostasis
Medical interventions in hemostasis are crucial for managing bleeding and clotting disorders, ensuring patient safety during surgeries, and treating emergencies. These interventions encompass a range of strategies and products designed to either inhibit or promote clot formation as needed. Key medical interventions in hemostasis include:
1. Anticoagulants
Anticoagulants are medications that slow the clotting process to prevent thrombosis, which is crucial for patients with conditions like coronary artery or cerebrovascular disease. These drugs, including warfarin, heparin, and newer agents like dabigatran and rivaroxaban, are essential in managing clotting risks but pose a significant bleeding risk during surgical procedures. Guidelines suggest discontinuing these medications before high-risk procedures and considering individual patient risk factors to balance the risk of bleeding and thromboembolic events. Bridging therapy with low molecular weight heparin can be used when the thromboembolic risk is high.
2. Hemostatic Agents
Hemostatic agents are used to promote clotting during surgeries or emergencies when conventional methods are insufficient. These agents include topical pharmacotherapies, sealants, adhesives, and absorbable materials. They are particularly useful in patients on anticoagulant therapy, as they help manage postoperative bleeding without systemic effects. Studies have shown that agents like tranexamic acid, bismuth subgallate, and autologous platelet concentrates are effective in reducing bleeding and promoting faster hemostasis. Novel agents, such as snake venom hydrogels, have also shown promise in rapidly controlling bleeding even in anticoagulated patients.
3. Surgical Techniques
Surgical techniques to control bleeding are critical in various medical procedures, especially in high-risk surgeries like cardiac operations. Techniques include the use of sutures, compression, and electrocoagulation. In cases where these methods are inadequate, hemostatic agents are employed to ensure effective hemostasis. The use of antifibrinolytic agents like aprotinin and tranexamic acid has been shown to significantly reduce blood loss during surgery. Additionally, advanced technologies and biomaterials are being developed to enhance hemostasis, particularly in challenging scenarios like internal non-compressible hemorrhage.
FAQs
1. What is the difference between primary and secondary hemostasis?
Primary hemostasis involves the initial formation of a platelet plug at the site of injury, while secondary hemostasis stabilizes this plug with a fibrin mesh through the coagulation cascade.
2. How is the hemostatic process regulated to prevent excessive clotting?
The process is regulated by natural anticoagulants such as protein C, protein S, and antithrombin, which help to prevent excessive clot formation and maintain a balance between clotting and bleeding.
3. Can diet or lifestyle affect hemostasis?
Yes, factors like vitamin K intake, hydration, and certain medications can influence blood clotting. Vitamin K, for example, is essential for producing clotting factors, while dehydration can increase blood viscosity, potentially affecting hemostasis.
4. What are common disorders related to defects in hemostasis?
Hemophilia, von Willebrand disease, and thrombophilia are common disorders that affect hemostasis. Hemophilia leads to excessive bleeding due to lack of clotting factors, while thrombophilia increases the risk of excessive clot formation.
5. How do external factors like medications or surgery influence hemostasis?
Medications like anticoagulants (e.g., warfarin, heparin) inhibit clot formation and can increase bleeding risks during surgery. Surgeons often manage this with hemostatic agents or adjust medication protocols before procedures.
6. What is the role of platelets in wound healing beyond clot formation?
Platelets not only form clots but also release growth factors that promote tissue repair and regeneration, playing a key role in wound healing after hemostasis.
7. How does hemostasis differ between individuals with normal clotting and those with clotting disorders?
In individuals with clotting disorders, the hemostasis process is often slower or incomplete due to deficiencies in clotting factors or platelets, leading to either excessive bleeding or clot formation.
8. What advancements in medical technology support artificial hemostasis?
Recent advancements include the development of synthetic platelets, nanotechnology-based hemostatic agents, and improved biomaterials for wound sealing, which enhance artificial hemostasis in patients with bleeding disorders.
9. Can environmental factors like temperature affect hemostasis?
Yes, cold temperatures can slow down the hemostatic process by reducing the activity of clotting enzymes and platelets, leading to prolonged bleeding in colder conditions.
10. How does the body naturally resolve clots after tissue repair is complete?
The body initiates fibrinolysis, where enzymes like plasmin break down the fibrin clot once tissue repair is complete, ensuring that blood flow is restored and clots don’t persist longer than necessary.